1.0 INTRODUCTION
Hazardous
Material Technologies Corporation (HMTC) was reatined
by Biological Controls to test the effectiveness of the
Microcon™ as an auxillary
ventilation system in filtering a simulated airborne pathogenic
organisms such as TB
bacillus and the AIDS virus. HMTC was asked to test the
Microcon’s ability to
control an aitborne concentration of responsible particulate of 1-3 micron
in diameter (see Appendix II for deatils information
of particulate characteristics).
The Microcon™ is a portable
self contained free standing air purification device.
It is designed to function as an internal ventilation
system capable
of both
filtration (99.97% of particulate > 0.3 microns in diameter) and disinfection
of pathogenic species. The unit can be operated at any one three (3) flow
rates.
The test method used consisted of generating an airborne
particulate concentration with in the test rooms, in the
range of 10-20 mg/M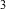 . The goal
of the test
was to determine the ability of the Microcon™ to remove airborne particulate
from
typical hospital environment under simulated operating conditions. All
testing was performed in soon to be renovated section of
Horton Hospital, Middletown, New York,
on December
22,1992. The two test rooms used in the test were a special isolation room,
and a patient residence room. . The goal
of the test
was to determine the ability of the Microcon™ to remove airborne particulate
from
typical hospital environment under simulated operating conditions. All
testing was performed in soon to be renovated section of
Horton Hospital, Middletown, New York,
on December
22,1992. The two test rooms used in the test were a special isolation room,
and a patient residence room.
-End of Section
1-
Next- Section
2 |

